Which of the Following Atoms Has the Smallest Radius
The most reactive of all elements is A chlorine. Which of the following correctly lists the five atoms in order of the five atoms in order of increasing size smallest to largest.
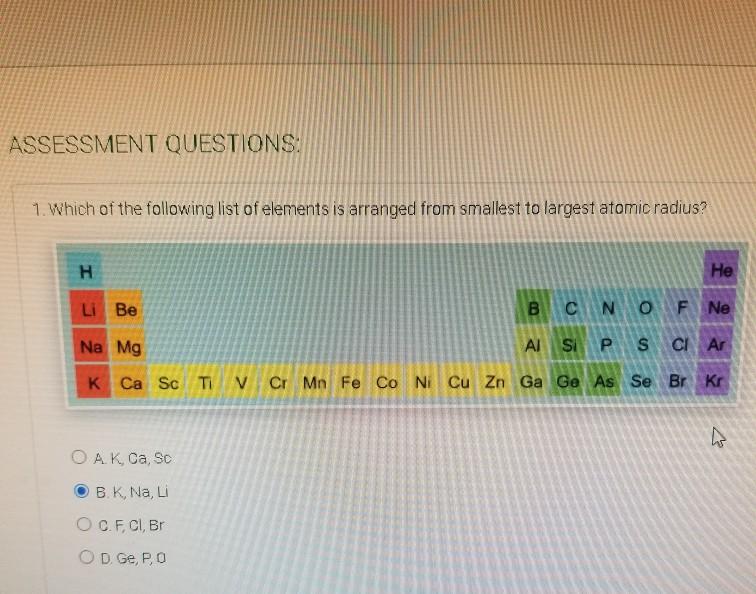
Solved Assessment Questions 1 Which Of The Following List Chegg Com
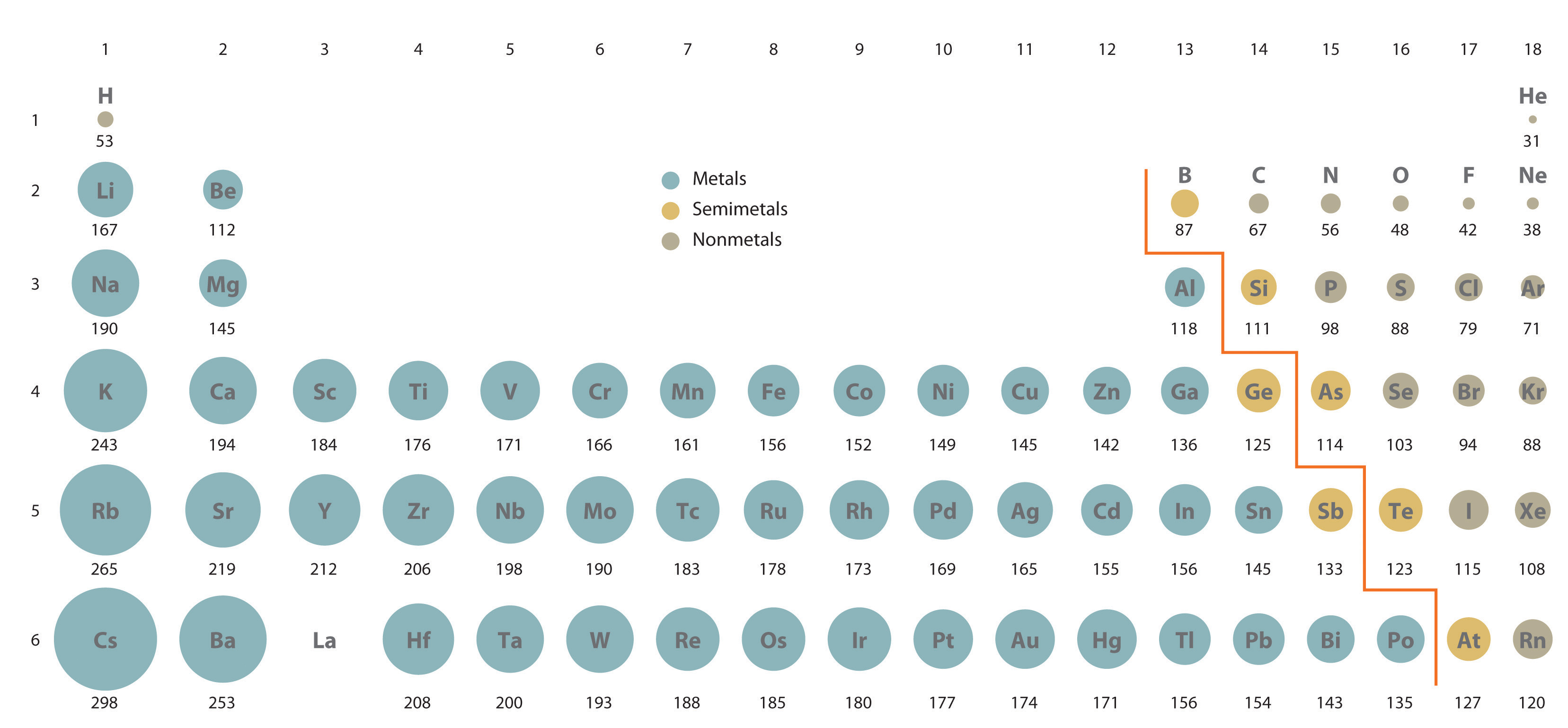
As can be seen in the figures below the atomic radius increases from top to bottom in a group and decreases from left to right across a period.

. A Cs b Rb c K d Na. Get 1-on-1 help from an expert tutor now. Atomic radius decreases along the period as nuclear charge increases and it increases down the group as shell is being added.
34 Which one of the following atoms has the largest radius. Which of the following ionsatoms has the smallest radius. Which of the following Period 3 atoms has the smallest radius.
119 rows Atomic radius in nanometers. A Element Z is further to the left side of the periodic table. Which one of the following atoms has the smallest radius.
A Co B Sr C I D Ca E Ba. This atomic mass represents the. The concentration of more protons in the nucleus creates a higher effective nuclear charge In other words there is a stronger force of attraction pulling the electrons closer to the nucleus resulting in a smaller atomic radius.
So all these elements belong to the 4th group and Kr has the smallest atomic radii as it lies across the period. An Introduction 7 Reactions In Aqueous Solutions 8 Chemical. À Ge In o Ga o Sn.
A Br b As c Ca d K. Based on this you could say. So an atom of helium is significantly smaller than an atom of hydrogen measuring by the radius of the electron cloud.
Of the following which gives the current order for the atomic radius for Mg Na P Si and Ar. Helium has the smallest atomic radius. Solve any question of Classification Of Elements And Periodicity In Properties with-.
Of the following which atom has the smallest atomic radius. Which of the following atoms has the smallest radius. For silver 47Ag108 how many neutrons are there.
As we move along a period radius decreases and as we move down the group radius increases so F have smallest radius. Which one of the following atoms has the smallest radius. A Na b Al c N d F.
Your email address will not be published. The distance between the center of the nucleus and the outermost electron is known as. B carbon C hydrogen.
Calcium potassium scandium titanium Which of the transition metals has the smallest atomic radius The atomic mass of titanium is 4788 atomic mass units. Thus D is correct. D A andor B.
View the full answer. B Element X is closer to the top of the periodic table. E B andor C.
C Element Z and X are probably in the same group. An Introduction 2 Measurements And Calculations 3 Matter 4 Chemical Foundations. Element Z is larger than Element X.
Elements Z and X are compared. Among the options Ba has largest radius. Atom radius decreases together you move throughout a duration from left come right and decreases together you move up a team from bottom come top.
Thus helium is the smallest element and francium is the largest. Advertisement Survey Did this page answer your question. 4 rows Which of the following atoms has the smallest radius.
Not at all Slightly Kinda. Which of the following atoms has the smallest atomic radius. Helium has actually the smallest atomic radius.
Which of the following atoms has the smallest radius. Mg S Sr Te Mg S Sr Te A. Which atom has the.
What has the smallest atomic radius out of hydrogen sodium and lithium. Which one of the following atoms has the smallest radius. This is mostly because the charge of the helium nucleus is twice as big as that of the hydrogen nucleus.
A Rb b Si c S d O. Elements Atoms And Ions 5 Nomenclature 6 Chemical Reactions. Which of the following atoms has the largest radius.
This is as result of trends in the regular table and also the reliable nuclear charge that holds the valence electron close come the nucleus. Select the correct answer below. A Nitrogen B Oxygen C Argon D Hydrogen E Chlorine 18.
1 POINT Which of the following atoms has the smallest covalent radius. Which of the following atoms has the largest radius. Among all the options ChlorineCl has smallest radius.
Of the following which atom has the smallest atomic radius Which element has the smallest atomic radius. Atomic radius increases going down the Periodic Table and going from right to left meaning that Fr Francium has the largest atomic radius and He Helium has the smallest. Required fields are marked Comment.
Helium has an atomic radius of 31 pm hydrogen has an atomic radius of about 53 pm. Na Get the answers you need now. A S B Na C Ar D Al E K Which of the following gases is monatomic.
Leave a Reply Cancel reply. Hydrogen has the smallest atomic radius - 25 pm.
Atomic Radius Trend Periodic Table Chemtalk

Atomic Radius Definition Types Variations In Periodic Table Videos

Question Video Determining Which Alkali Metal Has The Smallest Atomic Radius Nagwa

The Parts Of The Periodic Table
For A Given Period Which Is The Smallest Atom Socratic

Atomic And Ionic Radius Trends Ionic Radius Vs Atomic Radius Video Lesson Transcript Study Com
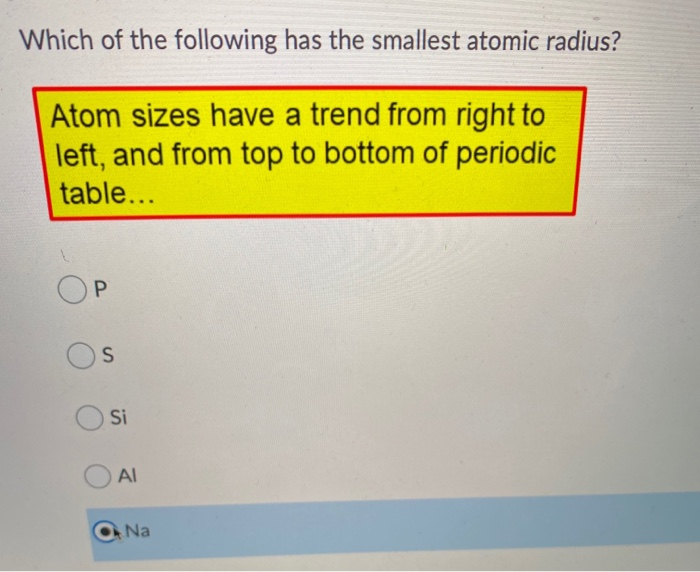
Solved Which Of The Following Has The Smallest Atomic Chegg Com

Periodic Trends Determine Which Atom Has The Smallest Atomic Radii Radius Johnny Cantrell Youtube

The Parts Of The Periodic Table

Atomic Size Atomic Radius Definition Variation In Periodic Table With Videos Of Atomic Radius
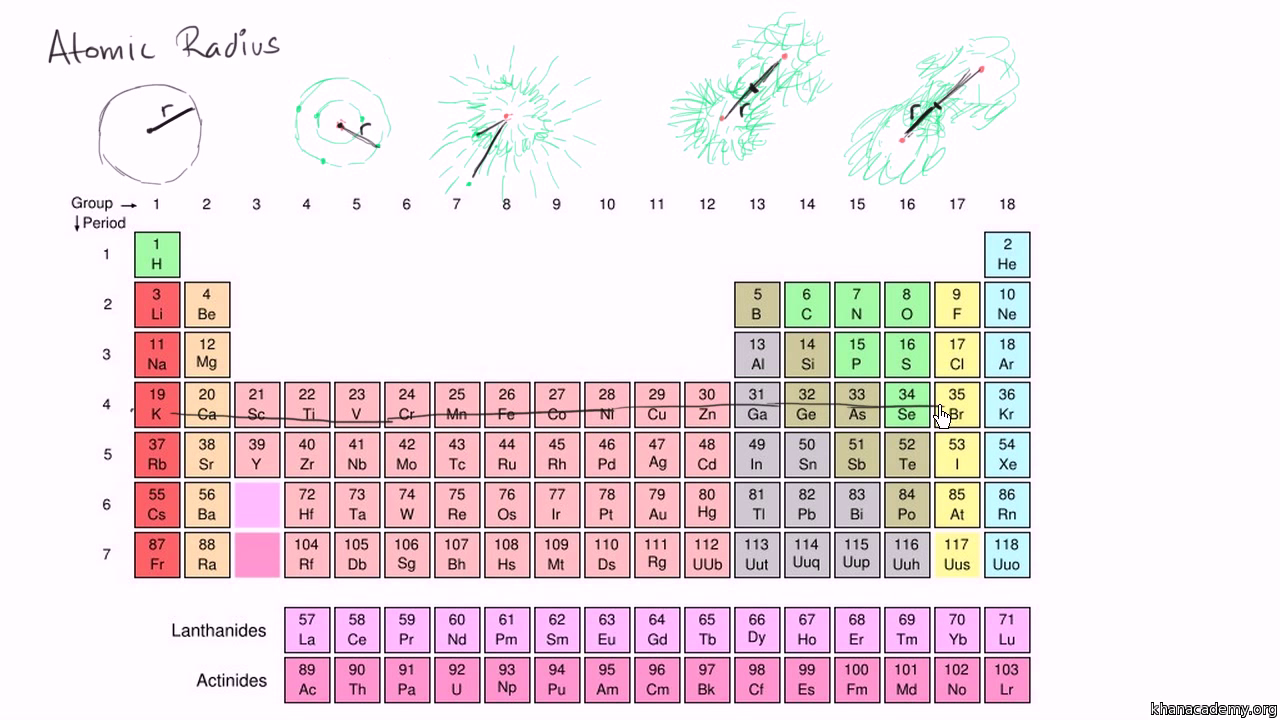
Atomic Radius Trends On Periodic Table Video Khan Academy

Chem To Go Lesson 11 Unit 3 Atomic Radius Ionic Radius Ppt Download

Solved An Atom Of Which Of The Following Elements Has The Chegg Com

Question Video Identifying Which Element Has An Atomic Radius Larger Than Aluminum Nagwa

Atomic Radius Trend Atoms Molecules Quiz Quizizz

Trends In The Periodic Table Chemistry Quizizz

Atomic Radius Definition Formula Example Video Lesson Transcript Study Com
Solved A In The Following Set Which Atom Has The Smallest Chegg Com

Comments
Post a Comment